Endotoxin Removal: From Bench to Process Scale
Published date: 08 December 2023
Endotoxin or lipopolysaccharides (LPS) are highly toxic components of the cell wall of Gram-negative bacteria and are often present in significant amounts in bacterial cell expression systems such as E.coli.
A number of methods have been adopted for the removal of endotoxin based on adsorption, in particular ion exchange chromatography. Although downstream processing can significantly reduce endotoxin levels in the product, efficient and cost effective removal of residual endotoxin from biopharmaceutical preparations remains a challenge.
Astrea Bioseparations has developed a novel affinity chromatography adsorbent, EtoxiClear®, that is highly stable, robust and non-toxic, with a high affinity for bacterial endotoxin and low protein binding. EtoxiClear® is a cost effective and scalable technology designed for use in endotoxin removal applications including process development, sample/buffer preparation and product polishing steps used during cGMP manufacture of biological molecules.
This application note describes the use of EtoxiClear® to effectively remove endotoxin from a purified immunoglobulin protein solution at both bench scale and process scale; utilising Astrea Bioseparations' 100 mm diameter Evolve® Process Column.
Materials & Methods
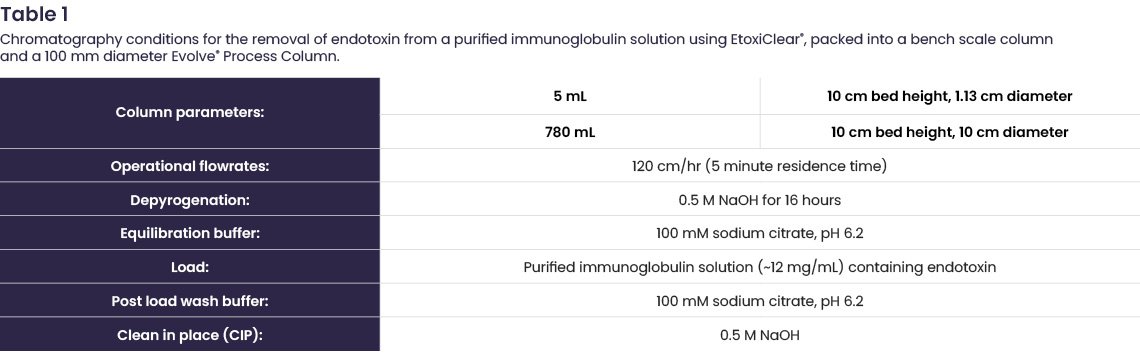
A purified immunoglobulin solution (~12 mg/mL) was determined to have an endotoxin burden. As a result, both a 5 mL column and a 780 mL Evolve® Process Column were packed with EtoxiClear®, using the guidelines in Table 1, to demonstrate high endotoxin clearance at different process scales. Prior to application the columns were depyrogenated for 16 hours using 0.5 M sodium hydroxide (NaOH). The NaOH was removed using ~1 column volume (CV) of water for injection (WFI) followed by equilibration buffer. Then 2 CV of the purified immunoglobulin solution was loaded onto each column and the non-bound fraction collected.
Results

Conclusions
EtoxiClear® provides an immunoglobulin protein recovery of >96% for the 5 mL column (bench scale) and >99% recovery using the 780 mL column (100 mm diameter Evolve® Process Column). There is a 3 log removal of endotoxin to obtain <1 EU/mL in the non-bound fraction, with less than 0.1 EU/mg protein for the Evolve® Process Column.
As a result, EtoxiClear® shows high performance and scalability for endotoxin clearance with low protein binding at both bench and process scale. The Evolve® Process Column is well suited for EtoxiClear® endotoxin removal applications and with the option to purchase a dedicated refresh kit to replace all the wetted parts of the column (including the acrylic tube), this column range provides significant cost savings whilst eliminating the need to prove endotoxin clearance from previous product and cleaning validation.

