Polishing matters: Why mAb purification is anything but one-size-fits-all
Published date: 14 April 2025
Monoclonal antibodies (mAbs) have become a defining force in modern medicine, with applications spanning oncology, immunology, infectious disease, and beyond. As demand for these therapeutics grows, the importance of robust, efficient purification processes has never been greater.
While much attention is paid to upstream expression and Protein A capture, the polishing phase of downstream purification is often where things get interesting—and more complex. This step, typically involving ion exchange, hydrophobic interaction, or mixed-mode chromatography, is critical for removing aggregates, host cell proteins, and other residual impurities that can affect product quality, safety, and stability.
The challenge is well known: no two mAbs behave the same. Differences in expression systems, charge, pI, stability, and impurity profiles mean that polishing strategies need to be tailored—what works well for one molecule might fall short for another.
That’s where a toolbox mindset comes in. Rather than of relying on a standard sequence, process developers are increasingly turning to a broader range of resins and chromatography modes to fine-tune purification based on each molecule’s unique behavior. Tools like chemical space mapping and viral clearance profiling are helping teams make data-driven decisions earlier in development, ultimately leading to more robust and scalable processes.
As mAbs continue to evolve and diversify, so must the technologies and strategies used to purify them. If you’re navigating the complexities of polishing, it’s worth knowing there are more options than ever—and that sometimes the most important step is simply understanding what is available.
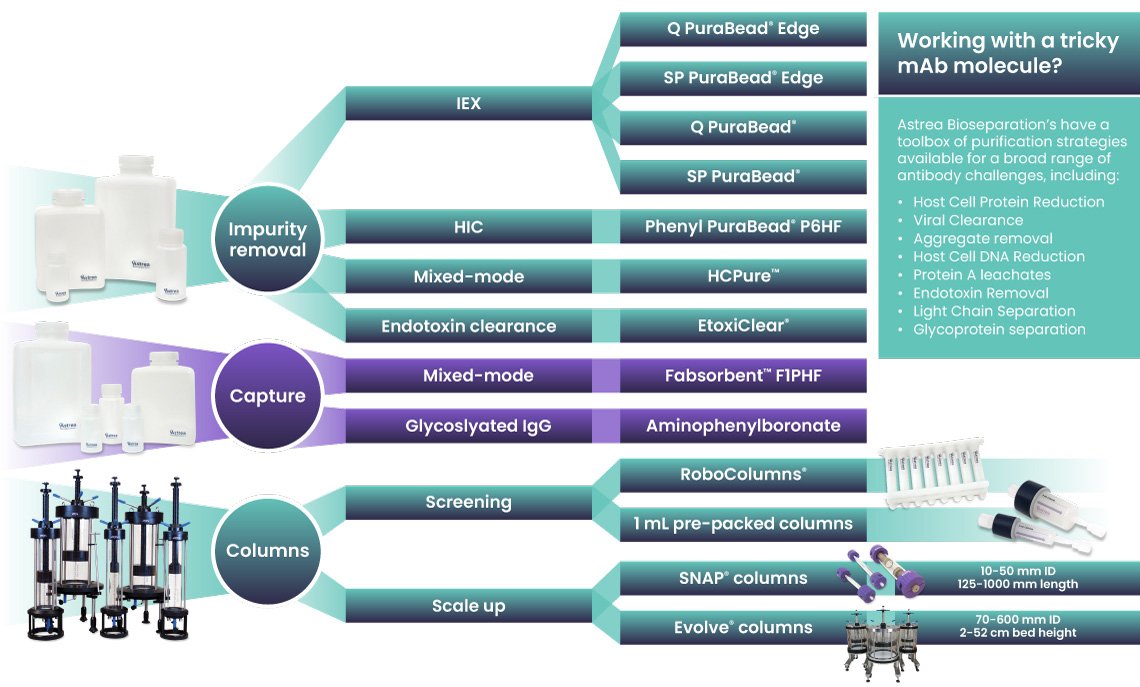
Explore our chromatography toolbox range for mAb and mAb derivatives, here. Curious to see how our resins perform in your process? Request your sample here.

