Protease Removal
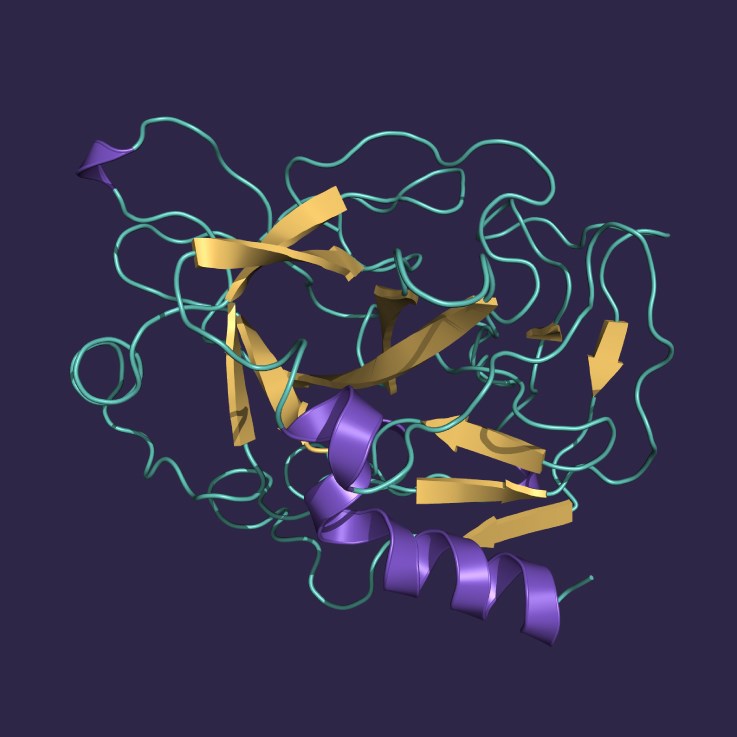
Proteases are enzymes that cleave peptide bonds in other proteins. During the downstream processing of biological products, the presence of active proteases is problematic as it may result in the creation of modified (truncated or clipped) product-related impurities, which are challenging to separate.
Therefore, the selective capture and removal of proteases is crucial for protecting the target biopharmaceutical product from degradation during purification and subsequent storage, potentially enhancing its shelf life.
Removal of serine proteases with p-Aminobenzamidine A6XL
p-Aminobenzamidine binds to the active sites of a broad array of serine proteases, including trypsin, thrombin, kallikrein, and chymotrypsin, as well as to certain sulfhydryl proteases and esterases such as papain and acetylcholinesterase. The selective binding properties of
p-Aminobenzamidine A6XL make it an ideal resin for capturing and purifying trypsin-like proteases, and for removing unwanted proteolytic activities in biological preparations.
Shop the Range