EtoxiClear®: A novel affinity adsorbent for the efficient removal of endotoxin
Published date: 12 December 2023
Endotoxin or lipopolysaccharides (LPS) (Figure 1) are highly toxic components of the cell wall of Gram-negative bacteria and are often present in significant amounts in bacterial cell culture expression systems such as E. coli.
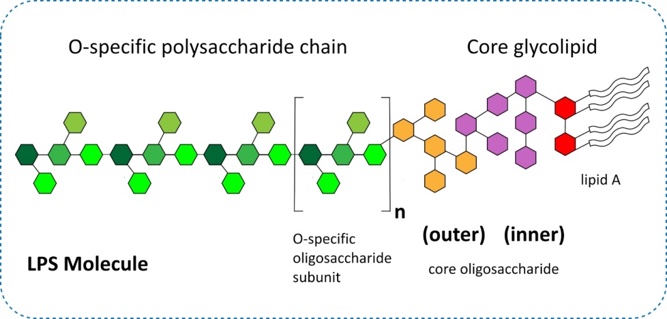
Fig 1. Schematic representation of Gram-negative bacterial endotoxin (LPS).
A number of methods have been adopted for the removal of endotoxin based on adsorption, in particular ion-exchange chromatography.
Although downstream processing can significantly reduce endotoxin levels in the product, efficient and cost-effective removal of residual endotoxin from biopharmaceutical preparations remains a challenge.
This technical poster addresses the issues of efficient removal of endotoxin from biological preparations. Specific reference will be made to a new synthetic ligand affinity adsorbent, EtoxiClear®, which exhibits high affinity for endotoxin, low protein binding and can be depyrogenated using sodium hydroxide.
The bi-dentate ligand, attached to Astrea Bioseparations' proprietary base matrix – PuraBead®, binds in a spatially selective and optimal manner to the LPS molecule with a binding capacity for endotoxin in excess of 1,000,000 EU/mL of adsorbent in a flow through chromatography mode (5 mL disposable EtoxiClear® column, loading at 120 cm/hr – 5 minute residence time).
A number of biomolecules with different isoelectric points have been used to demonstrate efficient protein recovery and clearance of residual endotoxin across the pH range. Protein recoveries in excess of 95% are achievable with endotoxin clearance to below 0.1 EU/mg protein.
EtoxiClear® has a high dynamic binding capacity for endotoxin and can operate in acidic to neutral conditions (pH 4.0 to pH 8.0) without a reduction in endotoxin clearance and typically maintaining >90% recovery of various proteins (up to 5 mg/mL concentration).
Performance & Scalability:
EtoxiClear® is available in a range of disposable column sizes including 5 mL (figure 1.) & 50 mL and can be readily packed into an Evolve® Bioprocess column. A standard bed height of 10 cm across the product range is recommended to provide effective process scalability.

Fig 2. 5 mL Disposable Column.
The disposable EtoxiClear® columns are designed for use in either process development applications or final polishing steps used during cGMP manufacturing of biological molecules, as well as the efficient removal of endotoxin from a range of buffers. The columns demonstrate excellent performance with comparable endotoxin clearance and protein recoveries across the range.
IgG protein solutions (~5 mg/mL), containing similar starting concentrations of endotoxin, were loaded onto the range of disposable EtoxiClear® columns, and both endotoxin clearance and protein recoveries were determined (Table 1).

Table 1. Performance data for endotoxin clearance from both the 5 mL column and 100 mm diameter Evolve® Process Column (780 mL) packed with EtoxiClear®.
Product comparison:
EtoxiClear® was compared against two commercially available competitor endotoxin removal products (IEX and affinity based membranes), using the manufacturers instructions. HSA protein solutions (10 mg/mL), containing similar starting concentrations of endotoxin, were loaded onto each product at a flow rate of 1 mL/min and both endotoxin clearance and protein recoveries were determined (Table 2).

Table 2. Comparison of EtoxiClear® versus other commercially available endotoxin removal products.
Varying endotoxin concentration:
IgG protein solutions (~5 mg/mL) containing a range of endotoxin starting concentrations (from low to high) were loaded onto EtoxiClear®, and both endotoxin clearance and protein recoveries were determined (Table 3).

Table 3. Protein recovery and endotoxin clearance results.
Results show that the EtoxiClear® adsorbent gives excellent endotoxin clearance (~0.1 EU/mg protein) and high protein recoveries (up to 100%), for protein solutions containing a range of endotoxin starting concentrations.
Endotoxin Removal from buffers:
A range of buffers, commonly used in cell culture applications, containing low starting concentrations of endotoxin (~20 EU/mL) were loaded onto EtoxiClear® and endotoxin clearance was determined (Table 4).
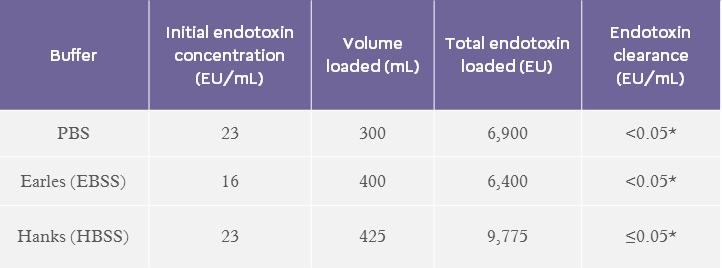
*equal to or below limit of detection BSS – balanced salt solution
Table 4. Endotoxin clearance results for various buffers.
Results show that the EtoxiClear® adsorbent gives excellent endotoxin clearance (≤0.05 EU/mL) from a range of buffers.
Endotoxin Removal – low protein binding:
EtoxiClear® has low protein binding and a wide range of proteins can be processed independent of their iso-electric point achieving high protein recoveries. Figure 3 indicates that typically >90% recovery is achieved for various model proteins spanning the pI spectrum.

Fig 3. Typical protein recoveries at neutral pH.
Endotoxin Removal – purified antibody fragment:
EtoxiClear® was used to remove residual endotoxin from an antibody fragment from an E. coli lysate partially purified using Fabsorbent™ F1P HF as a capture step (Table 5). Clarified cell lysate was loaded onto Fabsorbent™ F1P HF and the F(ab’)2 fragment eluted at pH 5.0. The resulting elution fraction was loaded directly onto EtoxiClear®.


Table 5. Endotoxin levels determined using a chromogenic endotoxin assay kit.
Fabsorbent™ F1P HF produced a high purity antibody fragment, with a >3.0 log reduction of endotoxin achieved using EtoxiClear®.
Endotoxin Removal – chromogenic (LAL) assays:
There are many commercially available endotoxin detection tests/kits to determine endotoxin clearance from protein solutions. However, if a chromogenic based test is used, it is recommended to include Glucashield® buffer to render the reagent insensitive to (1→3)-β-D-glucan interference which may be present in the sample.
HSA (9.1 mg/mL) and IgG (9.6 mg/mL) protein solutions, containing endotoxin, were loaded (1 mL) onto EtoxiClear® at 1 mL/min. The flow through fractions were collected and the samples analysed using a chromogenic based test (with and without Glucashield® buffer) and by Lonza Bioscience by LAL Kinetic chromogenic assay (Table 6).

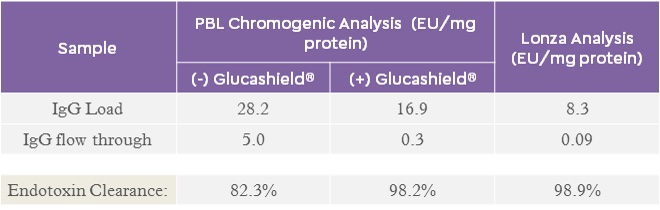
Table 6. Endotoxin clearance, from both HSA and IgG protein solutions, determined using a chromogenic endotoxin assay kit (± Glucashield® buffer) and by Lonza Bioscience in their endotoxin testing laboratory.
Inhibition of the β-D-glucan interference allows for more sensitive and more accurate determination of endotoxin removal comparable to the analysis performed externally at the Lonza Bioscience laboratory.
Summary:
- EtoxiClear® provides excellent endotoxin removal from a wide range of proteins across the pI spectrum, with recoveries that can be in excess of >95%, in a range of conditions from acidic to neutral pH.
- EtoxiClear® has a high capacity for endotoxin, in excess of 1,000,000 EU/mL of adsorbent, and is a available in a range of disposable columns (5 mL, 50 mL and 500 mL) with a standard bed height to enable process scalability.
- EtoxiClear® shows superior endotoxin clearance and protein recovery in comparison to other commercially available endotoxin removal products.
- EtoxiClear® gives excellent endotoxin clearance (~0.1 EU/mg protein) and high protein recoveries (up to 100%) for protein solutions containing a range of endotoxin starting concentrations.
- EtoxiClear® shows excellent endotoxin clearance (≤0.05 EU/mL) from a range of buffers commonly used cell culture applications.
- EtoxiClear® provided a >3.0 log reduction of endotoxin following the capture and partial purification of an antibody fragment by Fabsorbent™ F1P HF.
- The introduction of Glucashield® buffer to remove interference by β-D-glucans improved the sensitivity of the chromogenic assay and provided a more accurate determination of endotoxin clearance.

