Optimizing endotoxin removal with EtoxiClear®
Published date: 21 February 2025
As the biopharmaceutical industry continues to evolve, the need for high-purity therapeutics grows ever more critical. Among the key impurities in bioprocessing are endotoxins, bacterial contaminants, primarily derived from gram-negative bacteria, that are notorious for triggering severe immune reactions and can even compromise the efficacy of biologic drugs. Thus, effective endotoxin removal is a pivotal challenge in the downstream processing of biologics.
Astrea Bioseparations offers a solution with EtoxiClear®, a specialized chromatography resin designed to efficiently and selectively remove endotoxins from biologic preparations. In this article, we explore how EtoxiClear® can significantly enhance endotoxin removal, ensuring the production of safe, high-quality biologic products.

What makes EtoxiClear® unique?
EtoxiClear® specifically targets and polishes endotoxins from complex biological mixtures. This patented resin is optimized for high binding capacity and selectivity, offering several advantages.
EtoxiClear® is engineered to bind endotoxins at an exceptional capacity, significantly reducing the amount of endotoxin in your final product. This scalable solution, which can be used from bench-scale to commercial manufacturing, delivers consistent performance, ensuring high-purity products at every stage of production.
How does EtoxiClear® work?
EtoxiClear® resin is designed with a unique functional group that specifically targets and binds to the endotoxin core. During the chromatography process, the resin binds endotoxins while leaving the desired therapeutic protein or molecule largely unaffected. This selective interaction is key to maintaining the integrity of the target biologic and protein yield recovery, while achieving up to 99.99% reduction in endotoxin levels.
EtoxiClear® is not only effective for biologicals manufacture, it also shows excellent endotoxin clearance from a range of different buffers.
EtoxiClear® was compared against two commercially available competitor endotoxin removal products (IEX and affinity-based membranes), using the manufacturer’s instructions.
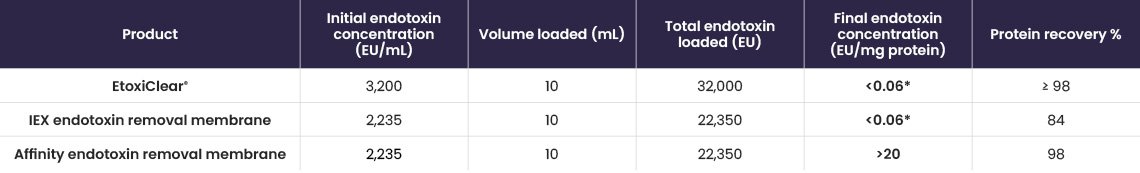
HSA protein solutions (10 mg/mL), containing similar starting concentrations of endotoxin, were loaded onto each product at a flow rate of 1 mL/min, and both endotoxin clearance and protein recoveries were determined. EtoxiClear® not only reduced the final endotoxin concentration to <0.06 Eu/mg protein but had over 98% protein recovery of HSA.
Key benefits of EtoxiClear® in bioprocessing
Ensuring the absence of endotoxins in biologics is a requirement for regulatory approval. EtoxiClear® enables manufacturers to meet the strict endotoxin limits imposed by health authorities such as the FDA and EMA, ensuring compliance and reducing the risk of costly delays in approval
Biologics production often involves complex workflows that require the removal of various impurities. EtoxiClear® has a binding capacity to endotoxins of >1 million EU/mL. It operates across a wide pH range (4-8) and can tackle endotoxins from various proteins, independent of their iso-electric point.
Mounted onto Astrea Bioseparations’ fast-flow PuraBead® agarose base matrix, EtoxiClear® is easy to both pack and run, with high flow rates and low back pressure. PuraBead® adsorbents are stable in up to 1.0 M sodium hydroxide which allows for stringent cleaning and sanitization protocols, so that it can be reused multiple times with long-lasting performance.
Recombinant proteins and monoclonal antibodies are particularly susceptible to endotoxin contamination during production. Likewise, proteins manufactured from precision fermentation using E. coli can have significant endotoxin challenges. EtoxiClear® effectively addresses this challenge, ensuring the final product is free from endotoxins.
In a case study, EtoxiClear® was used to remove residual endotoxin from an antibody fragment feedstock (E. coli lysate) purified using Fabsorbent™ F1P HF as a capture step.
Clarified cell lysate was loaded onto Fabsorbent™ F1P HF and the F(ab’)2 fragment was eluted at pH 5.0 and loaded directly onto EtoxiClear®.
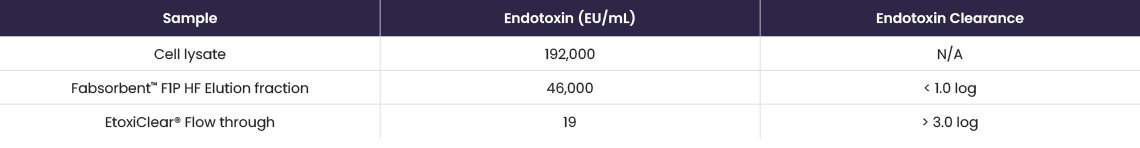
Fabsorbent™ F1P HF produced a high-purity antibody fragment, with a >3.0 log reduction of endotoxin achieved using EtoxiClear®
Why choose EtoxiClear®?
Astrea Bioseparations has a proven track record of working with large pharmaceutical manufacturers developing high-performance chromatography resins that address the critical needs of the biopharmaceutical industry. EtoxiClear® resin has been extensively validated to deliver consistent, high-efficiency endotoxin removal across a range of biologics, ensuring that manufacturers can meet the ever-growing demand for safe and pure therapeutics.
By leveraging the power of EtoxiClear®, manufacturers can streamline their processes, achieve regulatory compliance, and ultimately deliver the highest-quality products to patients.
If you are seeking an advanced solution for endotoxin removal, EtoxiClear® from Astrea Bioseparations offers an unparalleled combination of performance, efficiency, and scalability to support your biologic production needs.
Endotoxin contamination is a real industry challenge! Astrea Bioseparations makes endotoxin clearance easy with EtoxiClear®. Available in process-ready, pre-packed columns, ready to buy off-the-shelf from pre-clinical scale through to large-scale production.
For more information, click here or reach out to sales@astrea-bio.com.