HCPure™
HCPure™ is a mixed mode resin designed with the removal of Host Cell Proteins (HCP) in mind. With a unique operational space compared to other commercially available products, HCPure™ can provide alternative strategies for the removal of problematic HCPs and other process impurities.
Product overview
Product overview
HCPure™ is a mixed-mode resin designed with the removal of host cell proteins (HCP) in mind. This unique resin is composed of a proprietary mixed-mode ligand attached to PuraBead® 6HF agarose bead support. HCPure™ has a unique operational space compared to other commercially available products and can provide alternative strategies for the removal of problemative HCPs. Whilst selectively binding host cell proteins, HCPure™ can also remove host cell DNA, endotoxin, light chain IgG, and high molecular weight aggregates, to yield highly purified biological products with minimal target protein losses.
Due to its highly selective nature and ability to be tuned, by varying the chromatography conditions, HCPure™ removes impurities from a number of different expression systems, including, but not limited to CHO, E. coli, HEK 293, Pichia Pastoris, Saccharomyces Cerevisiae, and Spodoptera frugiperda (Sf9 and Sf21).
What are the key features of HCPure™?- Removes host cell proteins, host cell DNA, endotoxin, light chain IgG, and high molecular weight aggregates
- Works with broad range of feedstocks generated from multiple expression systems
- Fostering a unique operational space compared to other commercially available products, providing alternative HCP removal strategies
- Demonstrated consistent performance across a broad pH and conductivity ranges
- Suitable for applications such as antibody and antibody derivative workflows, recombinant proteins, lentivirus, and AAV
Host cell proteins: Challenges in mAb polishing
Optimizing the mAb polishing step is essential because no single solution fits all, and the process requires balancing trade-offs between yield, purity, time, and scalability. Variations in impurities from different expression systems, as well as differences in antibody size, charge, isoelectric point (pI), and stability necessitate tailored polishing strategies.
While the traditional method of mAb purification using Protein A capture, cation-exchange (CEX) bind-and-elute, and anion-exchange (AEX) flow-through steps is a good starting point for purification, it is not suitable for all mAb products. Challenges that must be addressed include protein instability in high-salt CEX elution and the presence of problematic HCPs that ion exchange alone fails to fully separate from the mAb product.
Unique operational space: Chemical space mapping
HCPure™ stands out for its unique ligand composition and distinct mode of action, as visualized in Figure 1, demonstrating the resins chemical space. Positioned to the right of the graph, it highlights its ability to leverage at least six different interaction mechanisms under varying conditions, offering superior versatility in protein purification. The graph highlights the differences of HCPure™ to other commercially available mixed-mode adsorbents, where the map demonstrates the comparable chemical properties, where similar binding resins cluster together. HCPure™ resin’s unique positioning emphasizes its distinct performance offering an alternative purification strategy for problematic HCPs.
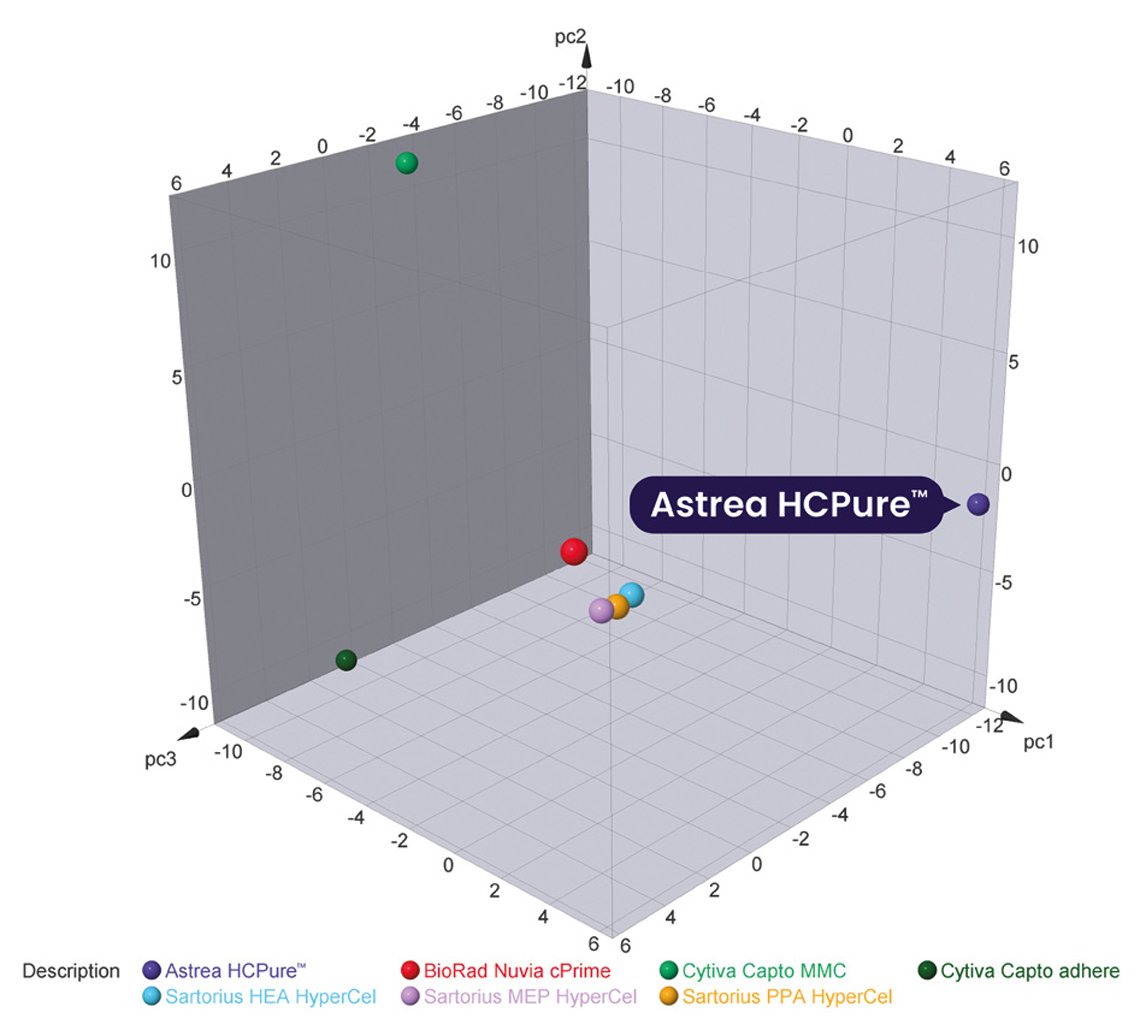
Figure 1. Chemical space diagram of commercial mixed-mode adsorbents.
In application: Antibody polishing
The removal of HCPs is a universal challenge across all antibody and antibody derivatives. HCPure™ is a mixed mode resin with a different operational space compared to other commercially available products and can provide alternative strategies for problematic HCPs.
HCPure™, a mixed-mode resin was evaluated for the impact of pH and conductivity on IgG purification versus another commercially available adsorbent. Heat maps, a predictive modeling tool used to visualise results, identify the most effective purification conditions, which, for HCPure™ showed consistent performance across a broad pH range. The importance of screening various resins is highlighted in Figure 2, where the heat map which display HCP clearance and IgG yield, where red regions indicate optimal performance.
The HCPure™ resin demonstrated consistent performance across a broad pH and conductivity range, maintaining high HCP clearance and IgG yield with minimal variation. In contrast, the competitor resin showed a strong pH dependency with optimal performance at pH 6, but with significant drops in yield at higher pH values, indicated by blue regions. This suggests that the ligand chemistry of the competitor adsorbent, interacted differently with the IgG compared to the HCPure™ resin. As a result, increasing pH caused the antibody to bind to a greater degree in the competitor product, reducing the recovery in the non-bound and therefore diminishing yield.
Overall, the HCPure™ resin provides an alternative strategy for problematic HCPs in antibody and antibody derivative manufacture. With stable performance, this resin provides a flexible option for HCP purification compared to alternative products.
_2025034-180610.jpg)
Figure 2. Heat map which displays optimal pH and conductivity for optimal HCP clearance (top row) and IgG yield (bottom row). Red regions indicate optimal performance and blue regions demonstrating poor performance. The heat map was used to determine optimal pH and conductivity for HCP log reduction and yield for an IgG produced in an HEK cell line.
To view the range of applications and technical notes available for HCPure™, visit the download section at the top of this page.
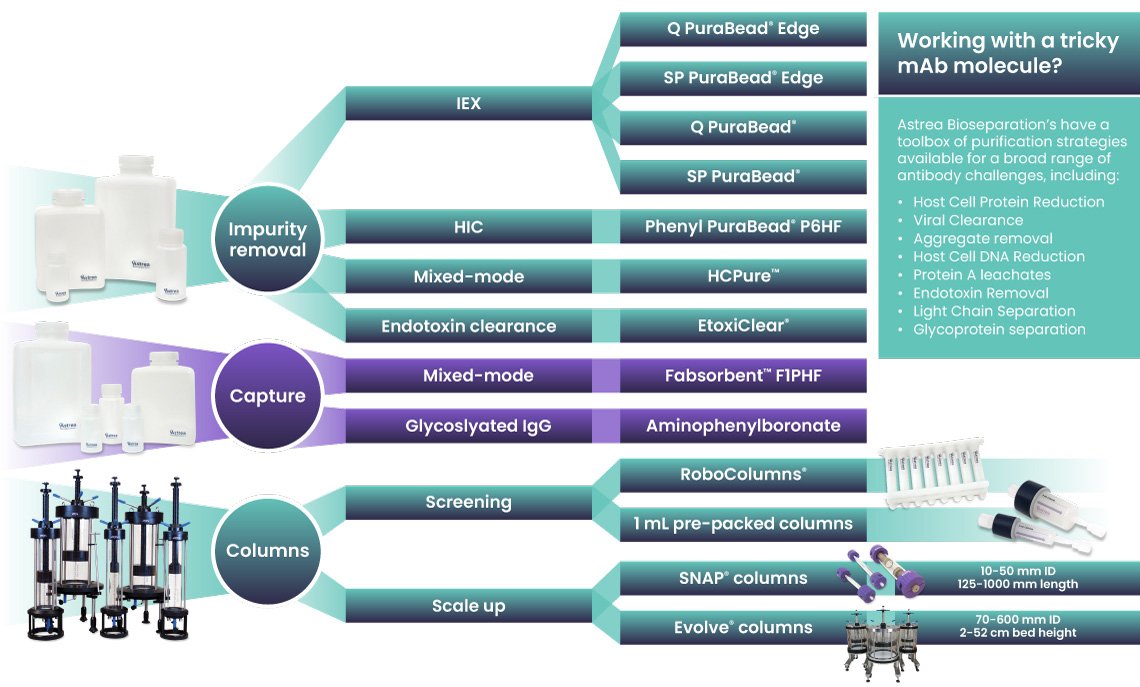
Astrea Bioseparations: A complete toolbox for antibody purification
As the demand for novel antibody formats grows, efficient screening of purification conditions becomes essential. Astrea Bioseparations has a broad range of purification resins, ranging from capture to polish, for mAb and other antibody formats. Using Astrea Bioseparations' toolbox approach, products, such as Fabsorbent™ F1P HF, HCPure™, Q PuraBead® and SP PuraBead® Edge, provide the market with additional choices for downstream processing workflows. Resins are provided in high-throughput screening formats to streamline discovery and purification. Combining smarter purification strategies and high-throughput screening options will help shape the future of biopharmaceutical development and commercialization of new drugs.
Simplified workflow integration and scalability
HCPure™ is designed for seamless integration into existing purification processes. Available in multiple column sizes for research or process-ready applications and bulk adsorbent slurry, ensuring scalability from lab-scale R&D to large-scale manufacturing.
HCPure™ is a solution trusted by industry players to ensuring safe, pure, and high-quality antibody products. It provides a powerful alternative over traditional monoclonal antibody capture & purification methods, helping manufacturers achieve efficiency, reliability, and compliance.
Product specifications
| Feature | Description |
|---|---|
| Ligand | Proprietary synthetic chemical ligand |
| Bead Size (µm) | 90 ± 10 µm |
| Matrix | PuraBead® PHF (Highly cross-linked 6% near-monodisperse agarose) |
| Recommended operational flow rates | Up to 600 cm/h |
| Operating pH | pH 4.0 to pH 8.0 (intermittent) |
| pH stability | Long term (3 months) pH 3 to pH 12 |
| Chemical stability | All commonly used aqueous buffers and co-solvents |
| Cleaning/sanitization | 0.5 to 1.0 M NaOH |
| Storage | 2–30°C, 20% ethanol |
Frequently asked questions
Frequently asked questions
What is HCPure™?
HCPure™ is a mixed-mode resin designed with the removal of host cell proteins (HCP) in mind. With a unique operational space compared to other commercially available products, HCPure™ can provide alternative strategies for the removal of problematic HCPs and other process impurities.
What are the key features of HCPure™?
- Removes host cell proteins, host cell DNA, endotoxin, light chain IgG, and high molecular weight aggregates.
- Works with broad range of feedstocks generated from multiple expression systems
- Fostering a unique operational space compared to other commercially available products, providing alternative HCP removal strategies
- Demonstrated consistent performance across a broad pH and conductivity ranges
- Suitable for applications such as antibody & antibody derivative workflows, recombinant proteins, lentivirus, and AAV
What formats is HCPure™ available in?
HCPure™ is available in pre-packed columns ready to plug into your research or GMP process. It is also available in slurry format.
What is the recommended operational flow rate for HCPure™?
The recommended operational flow rate is up to 600 cm/hr, depending on column size.
What is the recommended operating pH of HCPure™?
The recommended operating pH range for HCPure™ is 4 to 8 (intermittent).
How should HCPure™ be stored?
The recommended storage condition is 2-30°C in 20% ethanol.
How is HCPure™ regenerated?
Removal of any residual adsorbed material can be achieved by washing the column with 0.5-1.0 M NaOH. A contact time of 1 hour will normally suffice to ensure destruction of viable organisms, although up to 5 hours contact time may be required. No less than 5 column volumes are recommended. Once cleaning with NaOH is complete, wash with at least 3 bed volumes of equilibration buffer until the pH and conductivity of the column eluate is equal to that of the buffer entering the column. Complete this process prior to further use or storage in the storage buffer.
What types of biomolecules can HCPure™ be used with?
- Across a broad range of applications, such as antibody & antibody derivative workflows, recombinant proteins, Lentivirus and AAV
- Across a broad range of feedstocks such as CHO, E. Coli, HEK 293, Pichia Pastoris, Saccharomyces Cerevisiae, and Spodoptera frugiperda (Sf9 and Sf21)
What tools do Astrea Bioseparations have for antibody purification?

Astrea Bioseparations has a broad range of purification resins, ranging from capture to polish, for mAb and other antibody formats. Using Astrea Bioseparations’ toolbox approach, products, such as Fabsorbent™ F1P HF, HCPure™, Q PuraBead®, and SP PuraBead® Edge, provide the market with additional choices for downstream processing workflows. Resins are provided in high-throughput screening formats to streamline discovery and purification. Combining smarter purification strategies and high-throughput screening options will help shape the future of biopharmaceutical development and commercialisation of new drugs.
Related Products
Recently Viewed
There are no recently viewed products.
No video available.